Abstract
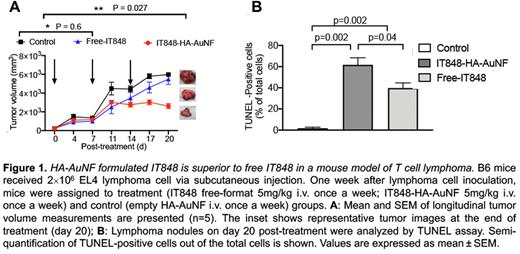
Hematologic malignancies account for one third of all cancers worldwide. Traditional chemo- or radiotherapy of these often highly aggressive cancers is limited by poor bio-selectivity and a narrow safety window, correlating with a risk for serious side effects and the development of refractory disease. Precision molecular therapeutics and highly targeted nanotechnological approaches can be employed as an alternative to overcome these challenges and successfully produce targeted anti-tumor responses, especially in the setting of disseminated disease. Herein, we report the formulation of a therapeutic nanocomposite comprising of a hyaluronic acid (HA)-coated gold nanoframework delivery system and encapsulated IT848, a novel small molecule with potent anti-lymphoma and -myeloma properties that targets the transcriptional activity of nuclear factor kappa B (NF-κB). We show that IT848 inhibits NF-κB activity through inhibition of DNA binding of all five NF-κB subunits. IT848 treatment of lymphoma and myeloma cell lines and patient samples inhibited proliferation and induced caspase-dependent and independent apoptosis. In addition to direct NF-κB inhibitory effects, IT848 treatment altered the redox homeostasis of cancer cells through depletion of the reduced glutathione pool, selectively inducing oxidative stress in cancer but not in healthy cells. IT848 significantly improved survival in an immunocompetent mouse model of multiple myeloma when combined with PD-1 inhibition, and correlative immune studies revealed that this clinical benefit was associated with suppression of regulatory T cell infiltration of the tumor microenvironment. We optimized the safety profile as well as the efficacy of IT848 by adapting HA-coated gold nanoparticles as an integrative platform for CD44-targeted delivery of IT848. Among a plethora of functionalized nanomaterials used for drug delivery, gold nanoparticles/nanoframeworks (AuNF) demonstrate excellent efficacy as drug carriers, photothermal or contrast agents, and radiosensitizers. Furthermore, easy synthesis and apt control over particle size, shape, porosity, and surface chemistry, together with their excellent biocompatibility make these particles ideal vehicles for small molecules such as IT848. HA-functionalized and IT848-loaded AuNFs were synthesized via a simple, reproducible, and inexpensive liposome-assisted technique that can easily be scaled up, a critical feature for clinical relevance. AuNFs were loaded with IT848 and surface functionalized with HA to formulate nanotherapeutics that were able to efficiently deliver the payload with high specificity to myeloma and lymphoma cell lines in vitro. In vivo studies characterized biodistribution, pharmacokinetics and safety of HA-AuNFs, and we demonstrated significantly superior specificity and efficacy of HA-AuNF formulated IT848 versus free IT848 in lymphoma mouse models (Figure 1). Our results indicate that active targeted delivery of IT848 using AuNFs via HA-CD44 ligand-receptor mediated endocytosis is a promising strategy to augment IT848 uptake by CD44-overexpressing tumor cells, mitigate rapid clearance of nanoparticles from the body and yield a highly desirable and sustained (as compared to free IT848) anti-tumor therapeutic effect. Our HA-AuNF-based drug delivery approach offers infrequent (weekly) low dose administration and robust efficacy with minimal toxicity risk. In fact, i.v. administration of IT848-loaded HA-AuNFs once or twice a week was not associated with any signs of toxicity, while traditional intraperitoneal administration of free IT848 twice a week over a 2-week period typically leads to ascites development in up to 50% of the recipients. Collectively, both our in vitro and in vivo results affirm that our AuNF system facilitates targeted drug delivery, improving the drug safety profile, and enhancing the efficacy of our novel NF-kB inhibitor molecule with minimal dosing. Our data therefore provide a strong preclinical rationale for the clinical development of HA-AuNF formulated IT848 as a therapeutic modality for the treatment of hematological malignancies. Additional studies are needed to develop curative treatment regimens based on synergistic, highly active, and well-tolerated combinatorial approaches with other targeted agents such as immune checkpoint inhibitors, hypomethylating agents, or CAR-T cells.
Disclosures
Tan:Incyte Corporation: Consultancy. Feinman:Takeda: Honoraria; GSK: Honoraria; Janssen: Honoraria; Merck: Other: Consulting; Cellularity: Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria. Siegel:COTA: Current equity holder in private company, Current holder of stock options in a privately-held company; GSK: Honoraria, Speakers Bureau; Takeda: Honoraria; Merck: Honoraria; Celularity: Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria, Speakers Bureau; Janssen: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal